
#Calculating formal charge of a molecule free
So this last oxygen, a free neutral oxygen has six valence electrons, from that, we're going We're going to subtract the number of valence electrons allocated to the bonded to nitrogen, well we see one, two, three, and then two more lone pairĮlectrons, so that is five, and so you have zero formal charge there. Periodic table of elements, and then from that, Has five valence electrons, we've seen that multiple times, you can look at that from the Now what about the nitrogen? Well we'll do a similar exercise there. On this oxygen atom, in this configuration of So six minus five isĮqual to positive one, and so the formal charge Would be one from this bond, one from that bond,Īnd one from that bond. Of the shared electrons, so half of the shared electrons Two lone pair electrons, and then it gets half

Number of valence electrons allocated to the bonded atom. We've seen multiple times, that is six, and then from that, we're going to subtract the And so the number of valence electrons in a free, neutral oxygen Now what about this oxygen here? Well we do the same exercise, I like to draw a littleīit of a circle around it. You can think of it as half the bond, youĬould say for each bond, it's going to be one electron 'cause it's half of the shared electrons, each bond is two shared electrons, but you're gonna say half of those, and then you have no lone pairs over here, so the number of valence electronsĪllocated to bonded atom, in the case of hydrogen here, is one, and so we are dealing with a formal charge of zero for this hydrogen. Now how many valence electrons are allocated to the bonded atom? Well one way to think about it is, draw a circle around thatĪtom in the molecule, and you want to captureĪll of the lone pairs, and you want to capture, Periodic table of elements, free neutral hydrogen So what's the number of valence electrons in a free, neutral atom of hydrogen? Well we've seen this multiple times, you could look at this on the So lets try and make sense of this by applying thisĭefinition of formal charge to the constituents of nitrous acid.
#Calculating formal charge of a molecule plus
Number of lone pair electrons, number of lone pair electrons plus one half of the So if we want to thinkĪbout the valence electrons that are allocated to a bonded atom, these are going to be the And so you're next question is, what does is mean to be allocated? Well, I will break up thisĭefinition a little bit. To subtract the number of valence electrons allocated, allocated to bonded, bonded atom.
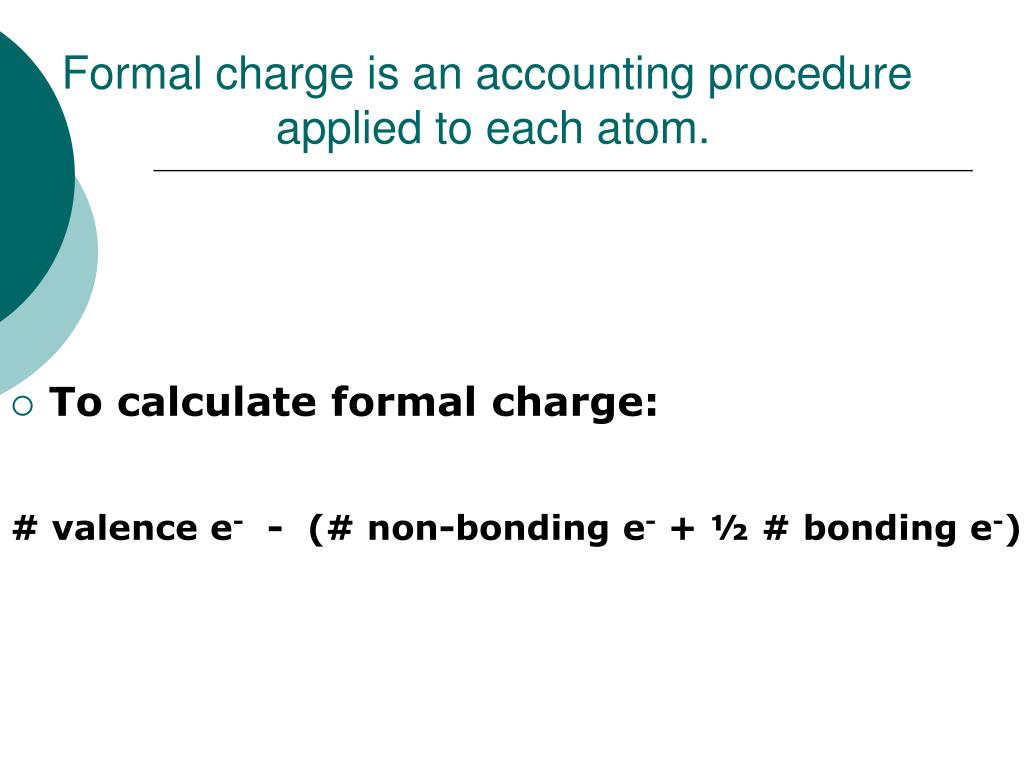
To calculate the number of valence electrons in free, in free neutral, neutral atom, atom. So the definition of formal charge, and we're going to do thisįor each atom in our molecule, for each atom, we're going To the resonance hybrid based on formal charge. Resonance structures for nitrous acid, but we'll think about which one contributes more These are both legitimate Lewis diagrams. We're going to calculate the formal charge on the various atoms in each of these resonance
So before going too deep into that, let's just give ourselves aĭefinition for formal charge, and then as practice, Molecule will contribute most to a resonance hybrid. Which resonance structures, which configurations of a
That we can calculate for each of the individualĪtoms in a molecule, and as we'll see in future videos, it'll help us think about The molecule as a whole, it's actually a number Is a tool that we can use as chemists to analyze molecules. The idea of formal charge, and as we will see, it In this video, we're going to introduce ourselves to


 0 kommentar(er)
0 kommentar(er)
